The United States Food and Drugs Administration is a respected regulatory body globally. When it gives a product, drug, or food item its approval or clearance, it’s usually a big deal for the brand. In fact, some nations do not give themselves much stress investigating the safety or effectiveness of a product once they can verify that it is FDA-cleared or approved.
For consumers, this badge is much more than a title or label; it often determines which product is worth their time and hard-earned money. When the product in question directly affects the body, such as an IPL device, nobody wants to overlook it. As such, FDA clearance or approval is no small consideration when shopping for the best IPL devices that can take care of your hair removal problem.
That said, is FDA clearance and approval the same? If not, what are the differences? And in the context of IPL hair removal, is the newly-released, industry-leading Ulike Air 10 IPL device FDA-cleared or FDA-approved? This article will answer these questions and more. However, let’s start by understanding what the FDA is and its mission.
What is the FDA? What Does It Do?
 The FDA is a US federal agency under the Department of Health and Human Services. Established in 1906, its main focus is safeguarding the health of the American people by ensuring and enforcing certain standards regarding the production, maintenance, and sales of foods, drugs, and electronic equipment used on the body.
The FDA is a US federal agency under the Department of Health and Human Services. Established in 1906, its main focus is safeguarding the health of the American people by ensuring and enforcing certain standards regarding the production, maintenance, and sales of foods, drugs, and electronic equipment used on the body.
The body examines a product or food’s claims, checks the associated risks, and determines whether its advantages outweigh its known risks. For IPL devices, the FDA will first examine its claims, safety features, associated risks, and overall benefits in light of the risks it poses before giving it a go-ahead. It is only when a brand presenting its product for approval can prove through evidence-based arguments that its product is effective and safe that the FDA can offer it its blessings.
This evidence-based argument involves trying the product on humans and animals and analyzing the results empirically. Of course, that’s tedious, time-consuming, and, in some cases, daunting. So, when a brand goes through all these and secures the agency’s approval or clearance, you can understand why they would make such a loud noise about it. It’s like undergoing a challenging course and coming out triumphant.
FDA & IPL: What’s the Importance?
 If you do a simple check of IPL devices online today, you will be amazed at the numbers. The hair removal niche is a multi-million-dollar industry, and there’s no doubt that Intense Pulsed Light (IPL) technology is its present and future. Consequently, many stakeholders are entering the market with different products. As a first-time IPL shopper, the FDA approval or clearance badge can help you weed out the pretenders from the real players in the industry. It narrows your choices to those with some degrees of precision, effectiveness, and safety.
If you do a simple check of IPL devices online today, you will be amazed at the numbers. The hair removal niche is a multi-million-dollar industry, and there’s no doubt that Intense Pulsed Light (IPL) technology is its present and future. Consequently, many stakeholders are entering the market with different products. As a first-time IPL shopper, the FDA approval or clearance badge can help you weed out the pretenders from the real players in the industry. It narrows your choices to those with some degrees of precision, effectiveness, and safety.
As you continue reading, you will discover that FDA approval or clearance may not be enough to determine which IPL device you should buy. However, it streamlines your choice to a group of reliable and relatively safe gadgets.
FDA Approval vs. FDA Clearance: What’s the Difference?
The next time you see the FDA badge displayed on any product, particularly an IPL, check to confirm the word used. That’s because FDA-cleared is not the same as FDA-approved.
The FDA generally classifies products into Class 1 (low risk), Class II (Moderate risk), and Class III (High risk). Only products belonging to classes one and two are qualified for the “FDA-Cleared” label. IPL devices are in the first two categorizations because of their moderate risk to humans. So, for products in these categories, all a company has to do to secure the designate, “FDA cleared) is to demonstrate that its product is at par with other cleared products from the FDA. That baseline product is called a predicate.
Prospective companies must submit a 510(k) premarket notification before obtaining the FDA-cleared label. Once evaluated and found consistent, the FDA awards the badge or label. This process may not require extensive clinical trials and multiple evaluating steps as requiring an “FDA-approved” badge.

FDA approval is a must for category III products. These often include drugs and medical instruments that pose a considerable risk to humans. Companies requesting this label must submit a premarket approval application that demonstrates that they have subjected such products or drugs to extensive clinical trials and that the known risks (if any) are insignificant enough to cause serious damage to the population.
So, if we want to differentiate the two based on their levels of complexity, FDA approval is more complex, tasking, and rigorous than FDA clearance. It requires real clinical trials, while clearance may not. It’s also primarily mandated for foods and drugs that have the potential to harm people if they are not safe.
Is Ulike Air 10 FDA-Cleared or FDA-Approved?
The newly-released Ulike Air 10 is FDA-cleared, meaning that it compares favorably well with already-cleared IPLs in safety and effectiveness. Stated differently, Ulike Air 10 is as safe and gentle on your skin as other devices that the FDA had earlier cleared and are being sold in the market.
You’ve got no reason to worry because its light will not burn your skin or cause any other damage. The caveat is that you, too, must use it as recommended. Already, the device is embedded with a smart sensor technology that helps you select the best light intensity for your skin type. However, you must ensure that you don’t abuse this device by over-flashing the lights.
Is Ulike Air 10 Clinically Tested?
 Of course! The new SHR mode of this pacesetting device is salon-motivated and included to provide a clinical result. From experience and customers’ feedback, it turns out that some hairs are stubborn and will not respond to traditional IPL device’s lights. Users had to either wait longer or use other methods to remove this stubborn hair.
Of course! The new SHR mode of this pacesetting device is salon-motivated and included to provide a clinical result. From experience and customers’ feedback, it turns out that some hairs are stubborn and will not respond to traditional IPL device’s lights. Users had to either wait longer or use other methods to remove this stubborn hair.
However, with this clinically motivated and tested mode, the energy output increases to an impressive 26 joules, guaranteeing that even the most obstinate hair will give way after two weeks. Clinical trials were conducted in a few places, confirming the effectiveness of this feature.
Do Dermatologists Approve of the Ulike Air 10?
Ulike enters a working agreement with board-certified dermatologists who liaise closely with their scientists to ensure that the products released are of the topmost quality to users. Some of these dermatologists, whose credentials can be confirmed online include Professor M.R Hambling, Dr. Gary Linkov, Dr. Davinn Lim, and two others. One of them, Dr. Linkov is a board-certified facial plastic surgeon.
All these eminently qualified physicians work in advisory roles to the Ulike Group’s scientists to ensure that quality remains the watchword. They each have excellent comments about the Ulike Air 10 and other devices when the subject is about safety, effectiveness, and design.
What is Special About the Ulike Air 10?
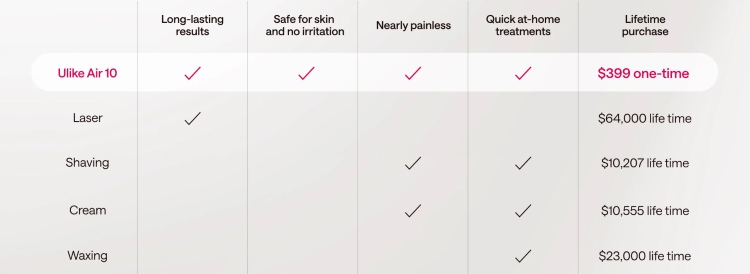 Going by the impressive features that Ulike Air 10 parades, it’s not out of place to say that it is the best IPL device in the world. Some of the outstanding features that make it the best globally include:
Going by the impressive features that Ulike Air 10 parades, it’s not out of place to say that it is the best IPL device in the world. Some of the outstanding features that make it the best globally include:
1. Dual Lights
In this release, the light source is doubled to ensure that users can cover more areas more effectively. No other IPL device features double light sources, and it explains why the handset outperforms others in areas such as time to give results and time to complete full body treatment.
2. SHR Mode
Ulike wanted to give customers salon-like results right in the comfort of their homes. As such, it included this feature, which works in tandem with the dual light mode to deliver an unmatched energy output of 26 joules. The model is suitable for dealing with spiky, stubborn hairs that often give most IPL devices a tough time.
3. NextGen Ice-Cooling Technology
It’s customary for IPL devices to become warm or hot after being used continuously for 30 minutes. When they get this hot, they can burn sensitive skin or cause redness and hyperpigmentation. Besides, when your hair removal device becomes hot, you will want to rush things to get over the discomfort quickly. Ulike addresses this challenge with its patented ice-cooling tech, which has been improved and fine-tuned for over ten years. The technology is clinically proven to keep users’ skin relaxed and safe for the treatment period.
4. Smart Skin Tone Sensor & Power Adjustment
Different skin tones tolerate different light intensities. Although the higher the light intensity, the quicker you are likely to see your hair diminishing, higher intensities can burn some skin types. The skin sensor takes away guesswork by matching users’ skin tones with the appropriate intensity level. It also changes the power mode as appropriate to ensure that you maximize the handset.
5. Four Power Modes
With the four power modes included in this device, you can use it from head to toe. If you don’t know which of the modes is best for the part you’re working on, the built-in sensor can help out by automatically selecting the most appropriate.
Conclusion
Ulike Air 10 is the company’s quickest and safest IPL device. It is FDA-cleared, affirming its safety and effectiveness as an at-home hair removal device.

 By ULIKEBEAUTY
By ULIKEBEAUTY









